VEST III
External Stenting and Disease Progression in Saphenous Vein Grafts Two Years After Coronary Artery Bypass Grafting: A Multicenter Randomized Trial
Study design & primary objectives
NCT02511834 (clinicaltrials.gov reference #)
Trial period: Oct/2015 - Oct/2019
Sample size: N=184
Participating centers:
- Krankenhaus der Barmherzigen Bruder, Trier, Germany
- German Heart Centre Berlin Clinic, Berlin, Germany
- West-German Heart Center Essen University, Essen, Germany
- Immanuel Klinikum Bernau Herzzentrum Brandenburg, Bernau, Germany
- Uniklinik Köln, Cologne, Germany
- John Radcliffe hospital, Oxford, UK
- Blackpool Victoria Hospital, Blackpool, UK
- Royal Papworth Hospital, Cambridge, UK
- University Hospital, Southampton, UK
- University Hospitals, Bristol, UK
- Medizinische Universität, Vienna, Austria
- Medical University, Innsbruck, Austria
- Sheba MC, Ramat Gan, Israel
- Rambam MC, Haifa, Israel
Principal investigator: Prof. David Taggart, Oxford, UK & Prof. Ivar Friedrich, Trier, Germany.
Design:
184 patients undergoing isolated CABG, using an internal mammary artery graft and at least 2 additional SVG, were enrolled to the VEST III study.
One SVG was randomized to an external stent (VEST), while one non-stented SVG served as the control.
The primary endpoint was SVG Fitzgibbon patency scale (FPS) assessed by angiography and the secondary endpoint was SVG intimal hyperplasia (IH) assessed by intravascular ultrasound (IVUS) in a pre-specified subgroup at 2 years.
Primary Objective:
To investigate the effect of external stenting on the progression of SVG disease 2 years post CABG.
Results summary
1. Baseline grafting parameters such as coronary artery diameter, graft’s length, TTFM and pulsatility index were well balanced between the VEST and control groups - (Table 1)
2. Fitzgibbon Patency Scale (FPS): FPS was significantly improved in stented versus non-stented SVG, with FPS I, II and III rates, respectively, 66.7% vs 54.9%, 27.8% vs 34.3% and 5.5% vs 10.8% (Odds Ratio 2.02, P=0.03) – (Table 2)
3. FPS was inversely related to SVG minimal lumen diameter (MLD), with FPS I, II and III SVG having an average MLD of 2.62mm, 1.98mm and 1.32mm, respectively (P<0.05) – (Table 2)
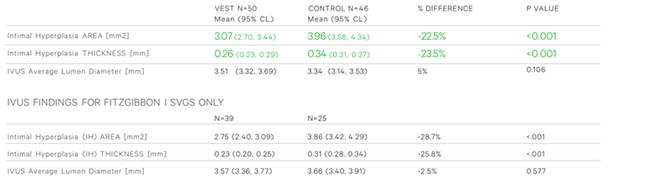
4. The externally stented SVG’s also showed significant reductions in mean IH area (22.5%; P<0.001) and thickness (23.5%; P<0.001) – (Table 3)
Main Conclusions
Two years after CABG, external stenting of saphenous grafts leads to improved Fitzgibbon patency and significantly reduces SVG disease progression by reducing both the area and thickness of intimal hyperplasia.
VEST III Publications
Name |
Link |
External Stenting and Disease Progression in Saphenous Vein Grafts Two Years After Coronary Artery Bypass Grafting: A Multicenter Randomized Trial |
Tables
Table 1: Baseline grafting parameters of study grafts
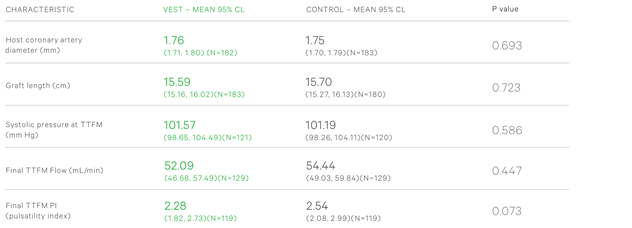
Table 2: Angiographic findings 2 years after CABG
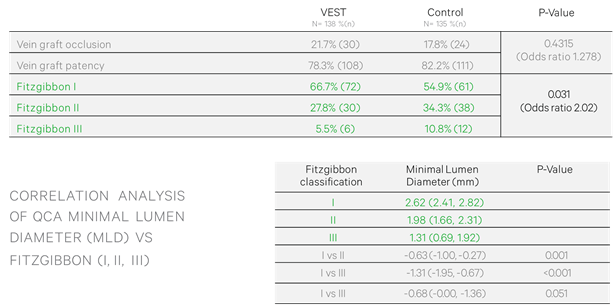
Table 3: IVUS based measurements at 2 years post CABG