Italian PMS study
External stenting of vein grafts in coronary artery bypass grafting: interim results from a two centers prospective study
Study design & primary objectives
NCT01860274 (clinicaltrials.gov reference #)
Sample size: N=102
Participating centers:
European Hospital, Rome, Italy
Mauriziano Hospital, Turin, Italy
Principal investigators:
Prof. Luca Weltert European Hospital, Rome, Italy.
Dr. Paolo Centofanti, Mauriziano Hospital, Turin, Italy
Design:
Prospective, all-comers study.
Patients scheduled for CABG with at least one vein graft.
Primary Objective:
Assess the clinical outcomes in a “real world” patients’ cohort in which the majority of the vein grafts are supported with VEST, and assessment of grafts patency at 6-12M post CABG.
Follow up:
- CT-angio at 6-12M
- Annual follow-up on MACCE for 10 years.
Results summary
84% of the patients had all SVGs supported with VEST and 16% (n=16) had at least one SVG with VEST and one unsupported.
This allowed for the creation of a within-patient reference group of non-stented SVGs for the analysis of VEST performance.
50% of cases were performed off-pump and 44% included sequential grafts.
VEST deployment was successful in 100% of cases
Follow-up Visit Results
1. CT angiography at 6-12 months, confirmed 98% patency for the VEST-SVGs (n=100) compared to 87.5% in the unsupported-SVG group (n=16).
2. Patency Rates at 6-12M, were as follows:
LIMA |
100% |
RIMA |
100% |
VEST |
98% |
Unsupported-SVG |
87.5%. |
Lumen Uniformity was demonstrated in 90% of the VEST-SVGs compared to 37% of the Unsupported-SVG.
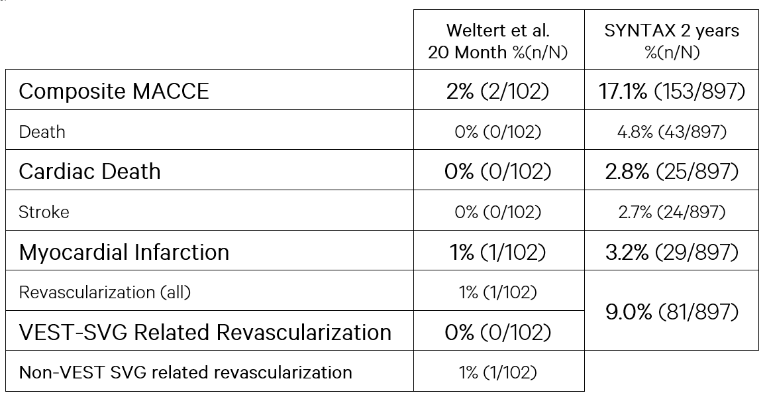
3. In-hospital MACCE included 2 patients (~2%). During the follow-up period of 20 months (range 6-54 months), the MACCE was 0%.
Comparison to historical data
Previous studies reported the effect of VEST in randomized controlled cohorts with specific pre-defined procedural settings which included only on-pump isolated CABG and external support of a single vein graft (within patient randomization design).
This is the first all-comers study in which VEST was tested in both off-pump and on-pump CABG and with either single or sequential grafting.
Main Conclusions
VEST is safe and effective in a real-world clinical setting that includes on- and off-pump CABG and a single or sequential grafting.
The excellent patency rates of 98%, compared to 87.5% reported in the reference group and to those described in the literature (70-80%) coupled with a very low MACCE rate (compared with the SYNTAX Study) at a mean follow-up period of 20 months, demonstrate the clinical benefit of VEST.
Italian PMS study
Name |
Link |
External stenting of vein grafts in coronary artery bypass grafting: interim results from a two centers prospective study |
